Parameter Mercury
Reduction of mercury contaminated waste water
Mercury is an extremely toxic metal. It is coming more and more under critical observation and is being regulated to extremely low levels in off-gas, waste and discharge water.
In the past mercury was used in multiple applications as in temperature measurements, electrical switches, high performance lamps, etching ingredients, chlorine-alkali electrolysis (amalgam process), gold mining and much more. Because of its multiple uses mercury is found in all waste streams. Our energy resources based on coal, crude oil or natural gas contains mercury.
During incineration of waste (WtE) or other fuels mercury evaporates and transfers to the flue gas. To avoid mercury release to the nature the flue gas can be treated dry or wet. Common technology at power plants is Wet Flue Gas Desulfurization (FGD) and the condensate washes the mercury out. Removing the mercury from the washing water (sometime called condensate) before discharging is an important topic to save humans brain in the future.
Mercury reduction:
Since multiple forms of mercury can be present in the same waste water stream, multiple removal techniques may need to be employed in series:
Filtration: Removal of colloid complexes; Oxidize organics
Adsorbent: Removal of zero-valent mercury; Removal of organo-mercury complexes
Functionalized Resin: Removal of mercury ions (Hg2+)
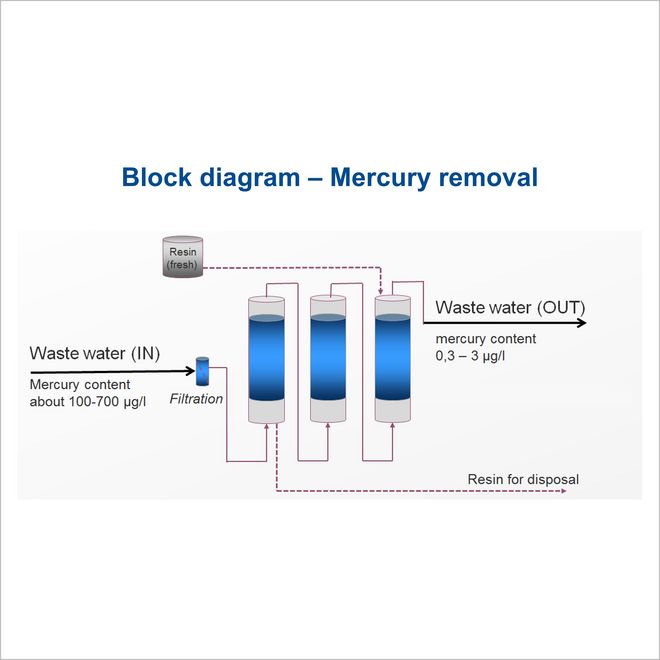
A typical mercury removal technology is separated in several steps. The steps are determined of the respective needs. For FGD condensate usually removal of colloid particle by filtration followed by selective working resin are used to reach the expected limits.
More information
Mercury in waste water
Mercury is found in municipal and industrial waste water. The content variate between 10 and 700 µg/l. Critical values are still in single digit values and represent a real ecological exposure.
Hazard because of mercury
Mercury is a very reactive molecule that exists in multiple forms. As the volatile zero-valent Hg (0), the charged ion Hg2+, and as organic-mercury compound, for example methyl mercury.
Because of its reactivity and volatility in water, mercury form complexes with many other ions, precipitate and accumulate for example in humans body. Mercury is present in air and water therefore animals and plants collect them and humans take it with their food.
Limits
Limiting the hazard of mercury contami-nation the authorities (for example in Germany: UBA – Umweltbundesamt) revised the limits for emitting Hg to the air of coal or waste fired power plants to 4 respective 7 µg/m³ annual (average).
Limits for waste water discharge are under discussion. Best Available Technology (BAT) is in evaluation. Industry expects limits between 0,3 to 3 µg/l waste water.
Filtration
Colloid particles, mercury complexes or from other sources, are removed by filtration. Depending on particle size and amount simple bag filters are used. In case particles less than 1µm ultra-filtration would be preferred and for smaller colloid materials nano filtration is necessary. Insitu oxidation of low amount of organic materials or special pre-treatment can be added at this stage.
Adsorption
Zero-valent mercury or organo-mercury complexes are able to be bound in a sulfidic reaction. Basically the waste water is loaded with NaS-solution or FeIIS-solution, the sulphur (S) dissolves in elementary form and Na or Fe creates a redox potential. Mercury is transferred in an ion form, reacts to HgS, and is bounded at the adsorbent and precipitate. The precipitated sludge is separated from the liquid by sedimentation and filtration. The filter cake needs to be disposed in a safe environment. Typical adsorbents are able to collect and bound 1 – 2 mass% of Hg.
Functionalized Resin
Functionalized ion exchange resins are available to remove different forms of mercury, for example methyl mercury and zero-valent mercury to very low levels; and also Hg2+. Ion exchange resins are used in industrial and acid-mining applications for metal separation.
Merry go round - procedure
Because of the low inlet concentration of µg/l and the very low discharge limits adsorbents and resins are breaking through at lower loads. Compare to adsorbents, resins operate in a closed vessel, where velocity and regime can be adjusted. To increase the load of the resin the merry go round procedure is applied. This procedure means, 3 or 4 columns are in serie and every column reduces the Hg content until the final column break through. At this point a fresh resin fill column is added at the front. Typical adsorbent resins are able to collect and bound until 5 mass% of Hg.
Request now
Do you have questions about delivery times, technical details or the realisation of individual systems & solutions?
Contact us! A member of staff will deal with your request and answer it promptly.
You can reach us by telephone at
+49 351 886 4600
Or by e-mail to
info@uit-gmbh.de